Residency at BIDMC is about discovery. Bright and curious minds cannot be constrained by what is already known; they find that residency is a continuous series of presentations of clinical mysteries that need to be solved, many of which do not have easy or available solutions.
We encourage our residents to discover their interests and talents, and to discover how the nervous system works, what goes wrong in neurological disease, and what we can do to improve the outlook for our patients. Our faculty are developing many of those solutions, and they welcome our residents as colleagues to join them on these voyages of discovery. Most of our residents choose to participate in a research project of their choice during their residency, and they present their results at our annual Resident Research Day. Faculty at BIDMC work on research problems in all subspecialties of neurology. Research themes range from single ion channels to the circuits that regulate behavior to the implementation of new therapies for patients. We have more than a dozen basic science research laboratories, investigating fields from Parkinson’s disease to epilepsy, sleep to autism, and brain tumors to peripheral neuropathies to stroke pathophysiology. In addition, our patients inspire robust clinical research programs in cognitive neuroscience, neuroimaging, neuropsychology, and non-invasive brain stimulation, autonomic physiology, muscle pathophysiology, and sleep physiology. As a clinical department, we have human-based research programs focusing on autism, Alzheimer’s disease, fronto-temporal dementia, memory disorders, stroke prevention, recovery from stroke, concussion, Parkinson’s disease, Huntington’s disease, Spinocerebellar Ataxia, ALS, peripheral neuropathies, autonomic disorders, epilepsy, brain tumors, multiple sclerosis, headache, and sleep disorders. In addition, the strengths of basic and clinical research approaches are being combined into several innovative translational research lines. The total NIH support for research in our Neurology Department in 2021 was $18,300,000, comparable to large neurology departments of entire universities (e.g., Cornell, Emory). Boston Children’s Hospital has a similarly outstanding level of support. To make these research opportunities easily accessible to our Neurology residents, we have a R25 program that has enabled over a dozen of our neurology residents to conduct postdoctoral research training during and after their residencies, helping launch their careers as successful physician-researchers.
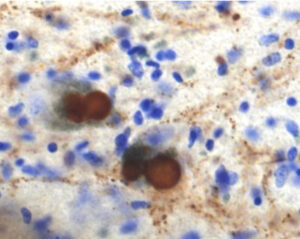
A section through the sacral spinal cord of a patient with Parkinson’s disease. Brown staining for alpha-synuclein is found in the lateral surface of the dorsal horn, which contains the pathways for bladder control.
Current research opportunities include:
NeuroNEXT The Network for Excellence in Neuroscience Clinical Trials, or NeuroNEXT, is a National Institutes of Health/National Institute of Neurological Disease and Stroke-funded clinical trials network of 25 sites across the United States (see www.NeuroNEXT.org). Beth Israel Deaconess Medical Center and Boston Children’s Hospital together serve as a single clinical site, supporting clinical trials in therapy and biomarker studies across the neurological disease spectrum. This network provides infrastructural support to help investigator-initiated clinical studies succeed. Our site also offers two funded training slots yearly to advanced fellows or junior faculty to support skill development and education in clinical trials research. C/T Academy The clinical-translational academy (C/T academy), offered through the Harvard Catalyst Clinical Translational Science Award (CTSA), offers a program to newly-minted fellows geared toward training in clinical translational research. In addition to a dedicated biostatistical course over the first summer, the program offers ongoing opportunities to learn about the intricacies and challenges of pursuing clinical research, with the ultimate goal of strengthening individuals to become more effective researchers. Weekly seminars are supplemented by a variety of other activities, including visits to the Food and Drug Administration and local biotechnology and pharmaceutical companies. Additional seminars are also held in conjunction with the KL2/CMeRIT Program below. KL2/CMeRIT Program This program, which is part of the broader Harvard Catalyst Clinical Translational Science Award, funded by the National Institutes of Health, supports training of advanced fellows and junior faculty in investigator-initiated translational research. Applications are typically submitted in the spring and awards made during the summer. In addition to providing 50-75% salary support, these awards offer a structured educational environment, including ongoing mentoring and opportunities to present their research to colleagues and input on preparing K-awards. Some of the sessions also occur in conjunction with the C/T academy offerings described above.
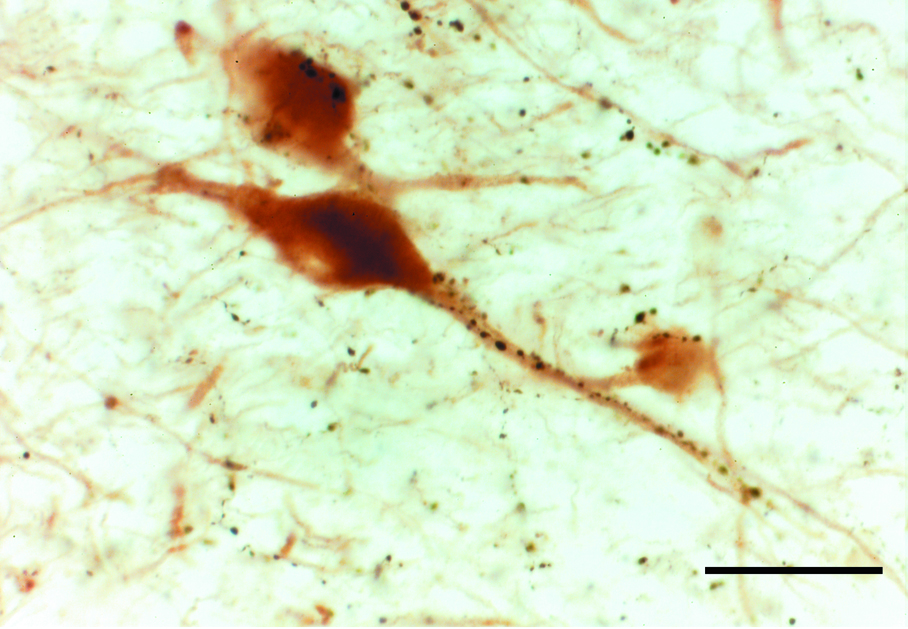
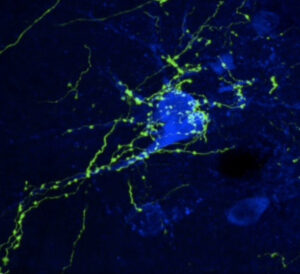
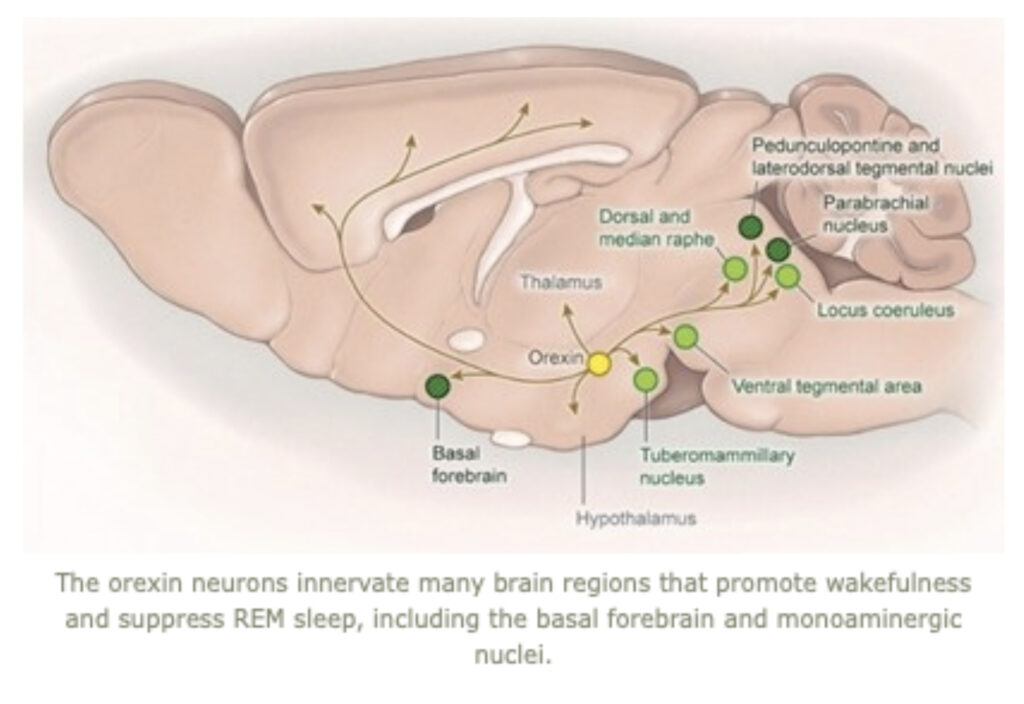
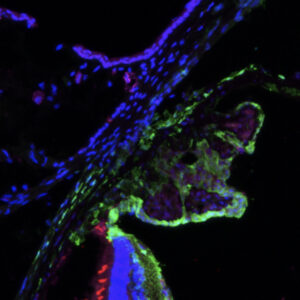
Neurons in the human locus coeruleus (brown, stained for tyrosine hydroxylase) are heavily innervated by axons and nerve terminals containing orexin (black boutons on soma and dendrites). In narcolepsy, the orexin-producing neurons are killed by an autoimmune process, and loss of their excitatory signals to the locus coeruleus and other brain regions results in sleepiness and poor control of REM sleep.
Harvard Catalyst Educational Offerings
As a clinical-translational “hub” for the Clinical and Translational Science Award (CTSA) at Harvard University, BIDMC residents, fellows and faculty are eligible to apply for educational offerings https://catalyst.harvard.edu/train/ that include biostatistics courses, IRB learning opportunities, grant-writing workshops, and more. These offerings are ideal to utilize in career development plans, and there is a wide variety to choose from to tailor to a number of research methodologies and approaches.
Faculty Investigators

Battelli, Lorella, Ph.D., Associate Professor of Neurology
The Battelli group investigates the cognitive neuroscience of vision, perception and attention in the adult human brain. They use noninvasive brain stimulation to study brain/behavior relation and to enhance brain functions in healthy subjects and in stroke patients. They use brain stimulation techniques such as transcranial magnetic stimulation (TMS), and direct current stimulation (tACS, tDCS and tRNS) coupled with behavioral training. The Batelli group uses brain imaging (fMRI and EEG) to measure the functional changes after brain stimulation. Her research lab focuses on the study of impaired cognitive functions in the diseased brain, and, more recently, they have been working to further test and refine their recently published tRNS-based treatment of visual cortical blindness, a severely debilitating visual deficit a consequence of cortical lesion to the visual areas after a stroke. They are running an NIH-registered clinical trial at the Beth Israel Deaconess Medical Center to collect more data on this promising procedure, and are using fMRI and EEG to measure cortical changes as markers of the behavioral improvement. The team is also testing other visual capabilities beyond the trained motion-perception task to examine whether the improvement transfers to other visual modalities within the damaged field. These experiments address the broader issue that is crucial to patients: does a procedure improve quality of life? Their innovative work might help identify how cognitive functions could be preserved/improved using noninvasive current stimulation as therapeutic treatment. In collaboration with other labs, they are also developing new protocols to treat Alzheimer’s Disease and fronto-temporal dementia.
PiN website: https://pinphd.hms.harvard.edu/people/lorella-battelli
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/32495
ORCiD: 0000-0001-9981-4028

Benitez, Bruno, M.D., Assistant Professor of Neurology
Dr. Benitez’ research is focused on the deep-molecular characterization of clinically well-defined cohorts of patients to generate a detailed molecular landscape of biospecimens of patients with neurodegenerative diseases. His group generate and integrate clinical and neuropathological data with genomic, transcriptomic, proteomic, and metabolomics datasets to identify novel molecular profiles associated with disease biology and clinical progression. They are also interested in the functional characterization of autophagy-lysosomal dysfunction in neurodegenerative diseases in cells and mouse models. They combine state-of-the-art approaches in human genetic discoveries (e.g. whole exome sequencing and GWAS data), functional genomic analyses in samples of neurodegenerative disease patients (e.g. single-nucleus RNA-sequencing, proteomic data, Bulk-RNA sequencing data) with in vitro (e.g. CRISPR/Cas9-corrected patient-derived cells) and in vivo (e.g. stereotaxic delivery of viral vectors) experiments. Their work aims to increase understanding of the etiology of neurodegeneration to enable molecular subtyping of patients with neurodegenerative diseases, reveal novel intermediate traits involved in neurodegeneration, provide strategies for cohort selection for testing therapeutic targets, and pave the road to identify disease-modifying targets for neurodegenerative diseases.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/56235
ORCiD: 0000-0002-2699-3878

Bouffard, Marc, M.D., Instructor in Neurology and Ophthalmology
The focus of the Bouffard Lab is on identifying the mechanism of idiopathic intracranial hypertension (IIH). To this end, their research includes a variety of approaches including hormonal analysis (this disorder almost exclusively affects overweight women of childbearing age), glymphatic imaging to determine whether interstitial fluid clearance is sub-normal, and both invasive and non-invasive measurement of intracranial compliance in IIH patients vs. controls. Our clinical research aims also include optimizing practical caregiving for patients with IIH and we have ongoing research investigating the prevalence and type of disordered eating behaviors in patients with IIH, the efficacy of nutritional interventions to achieve weight loss, and the efficacy of multidisciplinary IIH care clinics in the management of IIH.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/110684
ORCiD: 0000-0003-2353-0736

Buss, Stephanie, S., M.D., Assistant Professor of Neurology
Dr. Stephanie Buss is a practicing cognitive neurologist and Director of the Memory A2Z clinic at BIDMC. Her research focuses on investigating neurophysiologic mechanisms contributing to cognitive decline in Alzheimer’s disease. Her current work uses transcranial magnetic stimulation with electroencephalography (TMS-EEG) to measure how brain excitability predicts disease progression in early stages of Alzheimer’s disease.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/110703
ORCiD: 0000-0002-9912-063X

Fehnel, Corey, R. M.D., M.P.H., Assistant Professor of Neurology
Dr. Fehnel’s research focus lies at the intersection of aging, critical care neurology, and palliative care. Dr. Fehnel uses the tools of health services research to better describe outcomes among older persons with critical neurological illness. Using nationwide Medicare datasets he establishes trends in utilization, and identifies key determinants associated with favorable and unfavorable outcomes. As an attending neuro-intensivist at Beth Israel Deaconess Medical Center, the needs of his patients guide his research questions.
Dr. Fehnel’s previous work has examined prediction modeling of hospital readmission after acute ischemic stroke, as well as outcomes and location of long-term care among older patients receiving decompressive hemicraniectomy (removal of a portion of the skull) for severe ischemic stroke. He has also defined hospital characteristics associated with outcomes and quality of care for older patients with aneurysmal subarachnoid hemorrhage.
In addition to studying outcomes, Dr. Fehnel is particularly interested in intensive care symptom assessment and management among older brain injured patients receiving palliative care. His research has been funded by the American Academy of Neurology/American Brain Foundation and the National Institute on Aging.
Dr. Fehnel’s Lab Website: https://research.bidmc.org/corey-fehnel
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/39295

Frank, Samuel, M.D., Associate Professor of Neurology
Dr. Frank was the principal investigator for the Huntington Study Group (HSG) studies of deutetrabenzaine and the virtual Unified Huntington Disease Rating Scale. He has served as a site investigator for many observational and interventional trials related to Huntington’s Disease, Parkinson’s Disease and dystonia. He is the co-Chair of the HSG Executive Membership Committee. He was a member of the National Huntington Disease Society of America (HDSA) Board of Trustees and serves locally as the director of the HDSA Center of Excellence at BIDMC.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/92447

Fried, Peter, J., Ph.D., Assistant Professor of Neurology
Dr. Fried’s interests are focused on using TMS in conjunction with electromyography (EMG) and electroencephalography (EEG) This knowledge has the potential to improve public health by characterizing reliable and potentially modifiable markers of cerebral function that could be translated into novel therapeutic targets for interventions to improve cognition and reduce the risk of developing dementia.
Dr. Fried has a second line of applied research to evaluate the reliability and optimize the efficacy of noninvasive brain stimulation techniques such as TMS and transcranial electric stimulation as assessments of neurophysiology and the mechanisms of brain plasticity in humans.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/121647

Gibbons, Christopher H., M.D. Assistant Professor of Neurology
Dr. Gibbons is an Associate Professor of Neurology at Harvard Medical School and actively engaged in clinical/translational research and has published extensively on peripheral and autonomic disorders. His current areas of research include: 1) developing novel biomarkers for neurodegenerative disorders and 2) defining the mechanisms and pathophysiology underlying the development of neuropathy.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/67393
ORCiD: 0000-0003-0087-5336
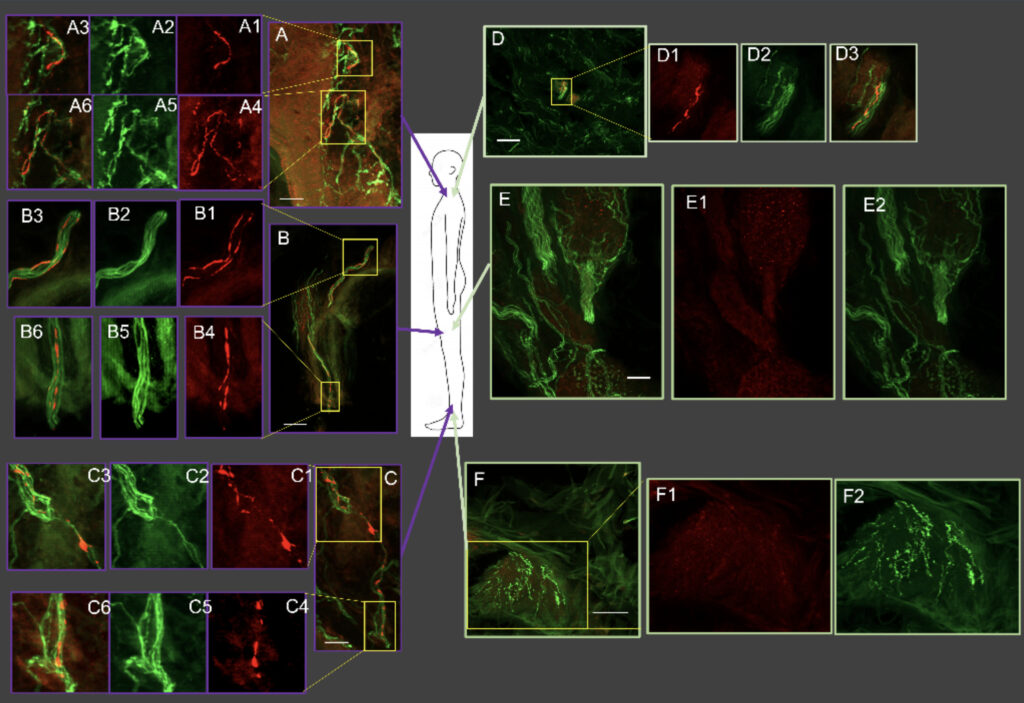

Goldenholz, Daniel, MD, Ph.D., Assistant Professor of Neurology
The Goldenholz Epilepsy + Data Science Lab is dedicated to using advanced data science tools to improve the lives of people with epilepsy. Some areas we focus on: non-EEG seizure forecasting, improving drug trials through simulation and statistical innovation, biosensors to detect high-risk patients, and automating EEG interpretation.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/27784

Greenstein, Patricia E., MB.BCh, Assistant Professor of Neurology
With advances in genomic medicine, there are emerging opportunities to diagnose rare diseases that patients have struggled with, for a long period of time. The integration of basic science into clinical neurogenetics is one of Dr. Greenstein’s areas of expertise clinically, and scientifically. She and her team have built up a fascinating clinic with regional referrals from the New England area to assist in the diagnosis of unknown diseases and to try to advance our knowledge about new phenotypic expressions of rare diseases.
Dr. Greenstein has collaborations with faculty at Children’s Hospital correlating the neurological phenotypes of galactosemia and phenylketonuria adults trying to understand the brain substrates using MRI Spectroscopy and MR tractography. She intends to expand this to the cerebellar ataxias, with research focused on understanding the mechanisms by which patients have this specific gait disorder.
Dr. Greenstein is the site PI for clinical trials for autosomal dominant spinocerebellar degenerations. In addition, she has collaborations with other investigators, including Dr. Veronique Vanderhorst, looking at more subtle phenotypic biomarkers in patients with ataxia. Dr. Greenstein is also planning to integrate this work with imaging approaches, including MRI resting state connectivity or tractography.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/5838

Monika Haack, Ph.D. Associate Professor of Neurology
While the peak of the COVID-19 pandemic has passed and life has returned to almost normal, millions of individuals worldwide have been left behind with Long COVID, also called Post-Acute Sequelae of SARS-CoV-2 (PASC). PASC will remain a major clinical and public health challenge in the years ahead, despite a reduction in the risk of developing PASC, due to variable coronavirus strains and a protective effect of vaccinations against the coronavirus. Among the most common symptoms remaining after three or more months following the initial or re-infection are fatigue, pain, cognitive impairments, post-exertional malaise, and sleep disturbances. There is an ongoing urgent need to better understand the risk factors, the disease mechanisms, and identify therapeutic targets. The Haack group investigates the role of sleep disturbances in the persistence and non-resolution of PASC, with a particular focus on the effect of sleep disturbances on innate and adaptive immune system aspects and pain. Sleep is known to play a critical role in infection risk, progression, and outcome. Sleep that is too short or disturbed (as manifested in difficulties falling asleep, frequent nighttime awakenings, waking up too early in the morning) compromises optimal development of B and T cell immune responses to pathogens, resulting in incomplete clearance of the virus, chronic infection, compromised immune memory development, and continuous cytokine signaling. Dr. Haack’s group have shown that short or disturbed sleep affects numerous pro- and counter-inflammatory pathways, including the nuclear factor (NF) k B pathway, the cyclooxygenase (COX) pathway, and the inflammatory resolution pathway. Many of the inflammatory signals have proalgesic properties and may contribute to pain in PASC, one of the most common symptoms in PASC, in particular in females. The Haack group uses comprehensive subjective and objective multimethod approaches to investigate the role of sleep in PASC.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/29715
ORCiD number: 0000-0001-6214-4294

Lee, Albert, Ph.D., Associate Professor of Neurology
Dr. Lee’s research is focused on the neural basis of memory and cognition, and on the role of the hippocampus and prefrontal cortex and their interactions with other brain regions. Their work involves studying animals as they learn, remember, imagine, navigate, solve, and decide things using a range of state-of-the-art systems neuroscience techniques. These techniques include:
– large-scale extracellular recording and imaging
– real-time processing and brain-machine interfaces
– intracellular recording
– optogenetics
– behavioral tasks in freely moving animals and virtual reality
– analysis of neural population data
– modeling (in collaboration with theoretical/computational neuroscience groups)
Individuals vividly recollect memorable moments from a vacation or imagine ways to rearrange their office while sitting at home. This ability to relive past experiences and simulate potential future scenarios can aid decision-making and also contributes to the richness of one’s inner life. The hippocampus is critical for both recall and imagination, but how is unknown.
To study this, the Lee group have developed a brain-machine interface (BMI) consisting of a real-time processor of large-scale extracellular recordings combined with an immersive virtual reality (VR) system. This enables them to train rodents to volitionally activate specific hippocampal firing patterns representing remote places. Such activation is a fundamental building block of recall and imagination, and the team is investigating the underlying mechanisms using the large toolkit available for rodent neuroscience.
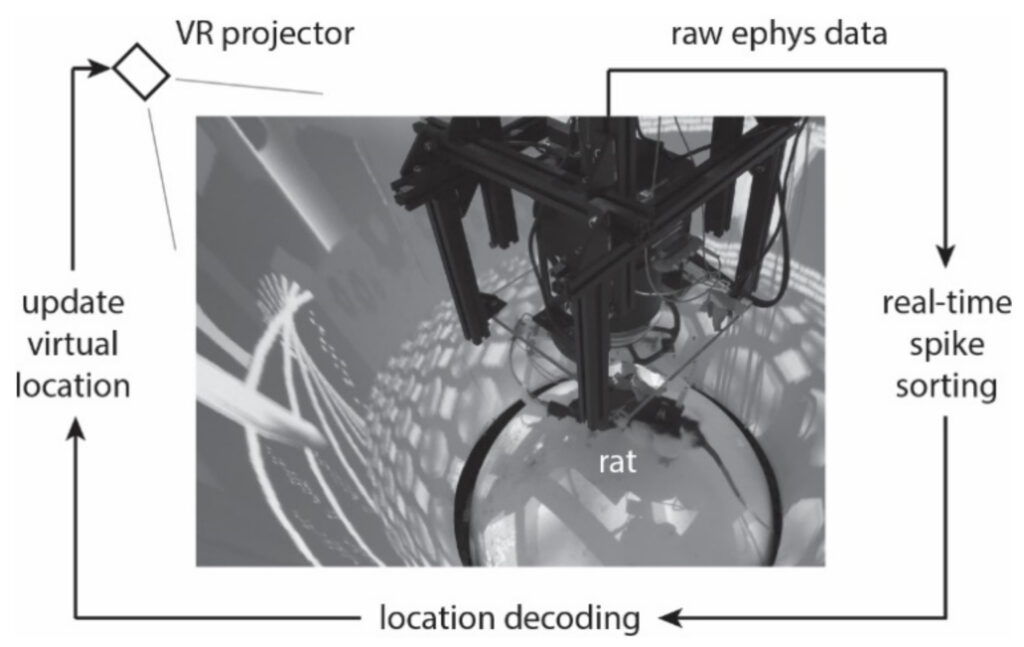
The Lee group also studies how memories of places visited are stored, events experienced, and other forms of information represented in the brain.
They have used a range of methods – intracellular recording in freely moving animals and animals in VR, extracellular recording in freely moving animals, and 2-photon calcium imaging in VR – to study the formation and structure of hippocampal memories of space. The group has discovered a simple mathematical expression that statistically describes how the hippocampus represents spatial environments under a wide range of conditions.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/211563

Mullington, Janet M., Ph.D. Professor of Neurology
Dr. Mullington’s area of expertise and research is in sleep deficiency and its associated pathobiological consequences for systems including inflammatory, blood pressure and heart rate, state-related neurophysiology, cognitive and subjective fatigue and mood. Her research team uses highly controlled approaches to induce experimental sleep deficiency of different durations (doses) to study effects of the build-up of deficiency in healthy sleepers, as well as the recovery process when sleep resumes. In addition, translational work of her group tests the efficacy of manipulating the timing and duration of sleep in order to affect health outcome indicators. Her group is interested in developing biomarkers of sleep deficiency.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/19266
ORCiD: 0000-0002-9156-9424

Press, Daniel, M.D., Associate Professor of Neurology
The Press research group is currently focused on developing new digital cognitive tools to detect and monitor the treatment of Alzheimer’s disease and related dementias. They use advanced analytics and a deep understanding of cognitive neuroscience to design and test tools that can quickly and accurately assess language, working memory/executive function, visuospatial function and declarative memory.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/1341

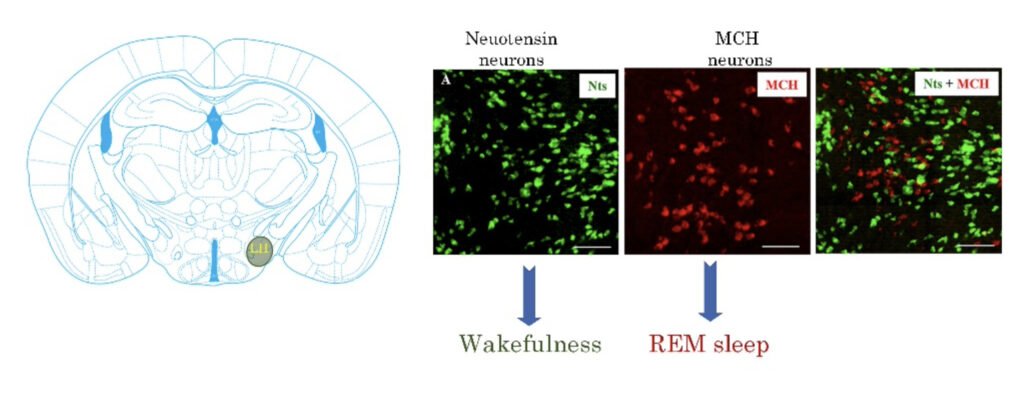
Ramalingham, Vetrivalan, Ph.D., Associate Professor of Neurology
The Vetrivelan Lab investigates the neural circuits that regulate sleep-wake states, with a special focus on two neurochemically distinct populations in the lateral hypothalamic area (LH) – the melanin-concentrating hormone (MCH) neurons and neurotensin (Nts) neurons. Their past work identified the wake-promoting role of LH-Nts neurons and demonstrated that these neurons are necessary to generate appropriate sleep-wake responses to environmental and physiological challenges. Adjusting sleep-wake behavior in response to such challenges may not only be of protective value but can also be vital for the survival of the organism. For example, while it is crucial to increase wake to explore a novel environment to search for potential threats and food sources, it is also necessary to decrease wake and reduce energy expenditure during the prolonged absence of food. In the absence of LH-Nts neuron activity, animals become maladaptive to these challenges. They currently investigate the downstream pathways and mechanisms via LH-Nts neurons optimize sleep and wake in challenging conditions. Similarly, their past work revealed that LH-MCH neurons specifically promote rapid eye movement (REM) sleep and identified the responsible downstream pathways and mechanisms. Furthermore, they also found that abnormal activity of these neurons contributes to the pathophysiology of narcolepsy with cataplexy (NT1), a sleep disorder affecting 1 in 2000 individuals worldwide. They found that activating MCH neurons increases the incidence of cataplexy (abrupt loss of muscle atonia similar to paralysis triggered by positive emotions) in animal models of NT1 while pharmacological blockage of MCH receptors led to near-complete suppression of cataplexy for 3-4 hours, highlighting a new therapeutic benefit option for the treatment of NT1 in humans. They are currently investigating the specific mechanisms and pathways through which MCH neurons contribute to the NT1 pathophysiology and the therapeutic benefits of MCH receptor antagonism. Dr. Ramalingham’s group takes a systems neuroscience approach and use a wide variety of classical (anatomical tract tracing, electrophysiology, lesions, pharmacological) and genetic techniques (optogenetics, chemogenetics, photometry, conditional genetic knockout/lesions) to investigate these research questions.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/63375
ORCiD: 0000-0002-6376-3641

Rutkove, Seward, B,, M.D. Nancy Lurie Marks Professor of Neurology
The Rutkove lab focuses on human and animal translational research for the development of novel diagnostics and therapeutics to help treat neuromuscular disorders and improve muscle and nerve health. Disorders of interest to his lab include serious conditions such as amyotrophic lateral sclerosis, muscular dystrophy, and nerve injury to milder problems, such age-related muscle wasting and changes due to disuse. They utilize and develop several different technologies with a focus on advancing electrophysiological techniques, including electrical impedance myography and nerve and muscle excitability testing and quantitative ultrasound techniques. They also have a strong interest in electrical muscle stimulation. Finally, they also have smaller but abiding interest in disease pathogenesis and testing new therapeutic agents that can improve function and health. Many of these tools are still being refined, and thus their research continues to be an iterative process in which early versions of engineering concepts are applied, data collected and analyzed, refinements made and testing repeated. Like all science, the process of discovery takes place in irregular patterns: many small steps before an unexpected greater leap.
In addition to having extensive support from the National Institutes of Health for their work, they have also been pursuing NASA-funded research, using their technologies to better understand the mechanisms and severity of change in microgravity (as would occur during space flight) and while living on the Moon and Mars. Current studies include human and animal investigations specifically understanding the impact of reduced gravitational effects on muscle, nerve, brain, and bone in both humans and animals.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/28388
ORCiD: 0000-0002-6375-3312

Saper, Clifford B, M.D., Ph.D., James Jackson Putnam Professor of Neurology
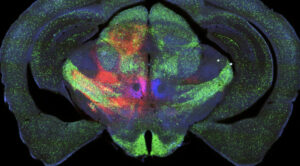
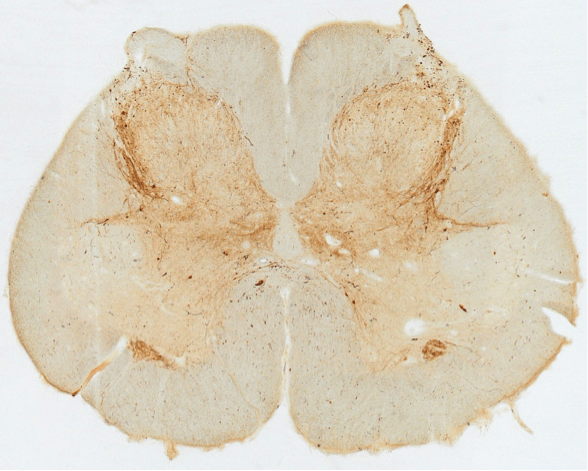
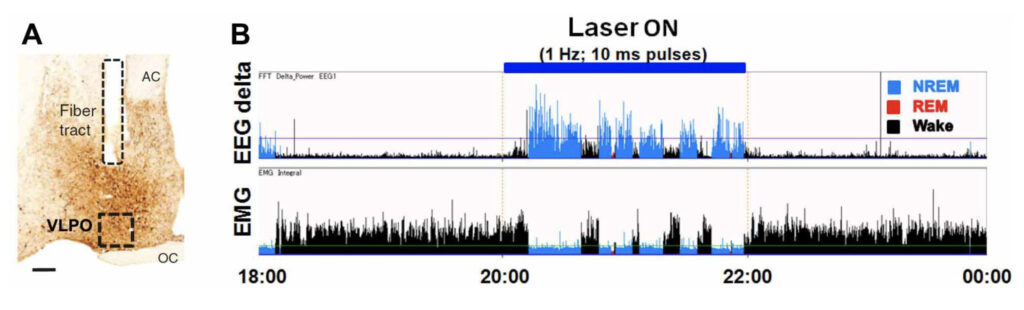
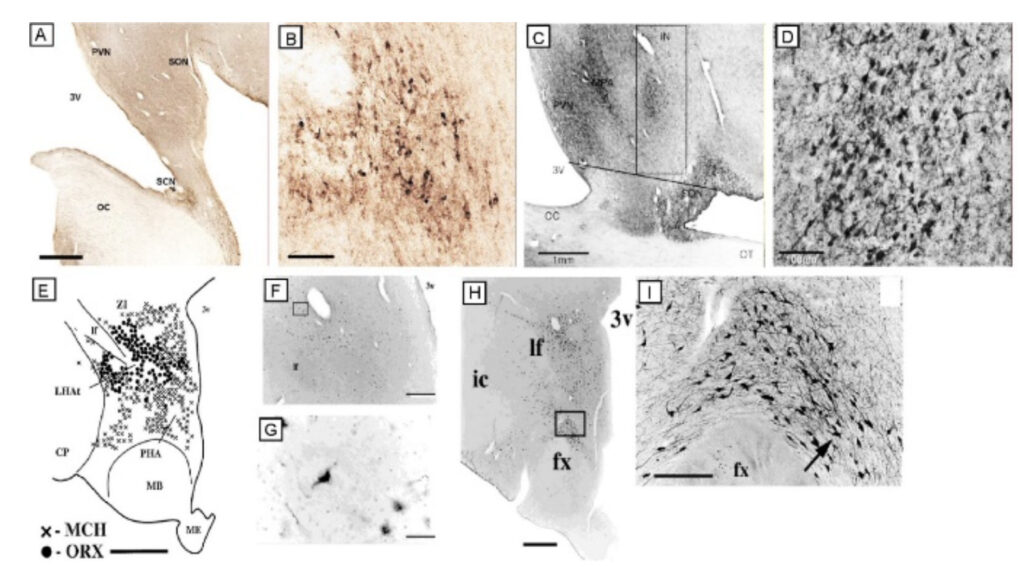
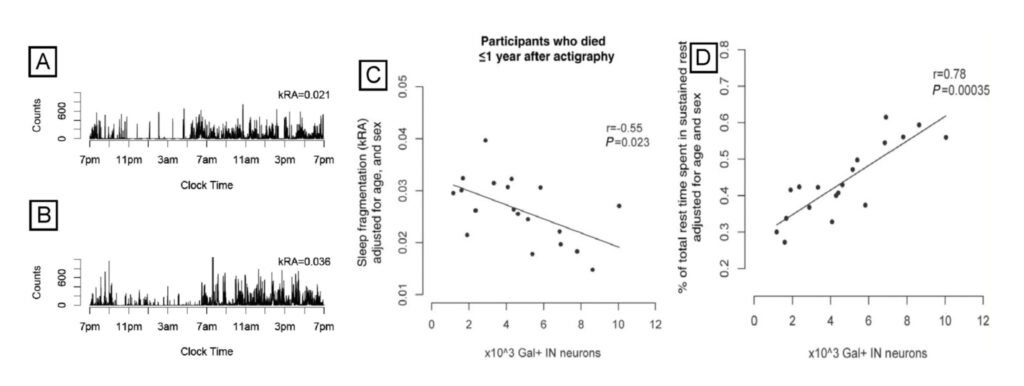
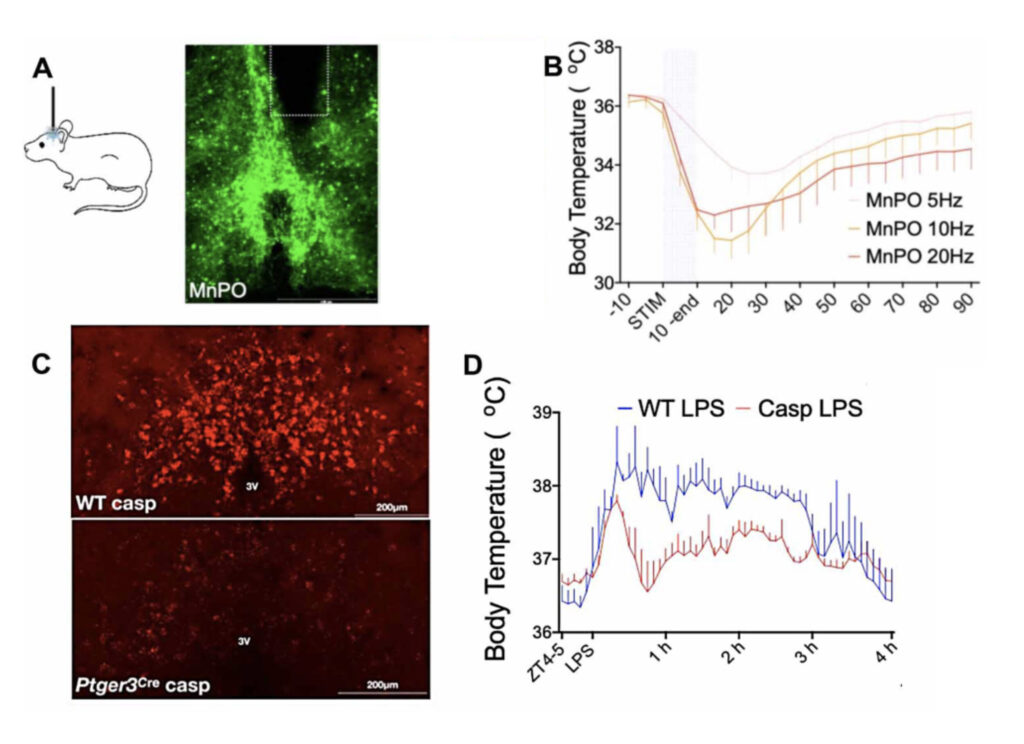
The Saper lab studies the brain circuitry that regulate wake-sleep and circadian rhythms and associated functions such as thermoregulation, energy metabolism, and stress responses. His team uses genetically targeted approaches such as optogenetics, chemogenetics, calcium imaging, spatial transcriptomics, and tracing synaptic connectivity in transgenic mice. They study some of this same chemically identified circuitry in the post-mortem human brain, both in health, and how this is affected by neurodegenerative disease. Recent examples of their work include: 1. Identifying the ventrolateral preoptic nucleus as a key cell group for promoting sleep both in mice (Fig. 1) and 2. Examining related wake sleep circuitry in human brains (Fig. 2). 3. Showing that loss of neurons in the ventrolateral preoptic nucleus in aging correlates with increased sleep fragmentation (Fig. 3). 4. Identifying neurons in the median preoptic nucleus that play a central role in thermoregulation, including producing fever responses (Fig. 4).
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/17796

Scammell, Thomas, E, M.D. Professor of Neurology
Research in the Scammell Lab focuses on the neurobiology of sleep and the neural basis of narcolepsy. Narcolepsy is caused by an extensive and selective loss of the hypothalamic neurons that produce the orexin neuropeptides(also known as hypocretins). This cell loss generally occurs in the teens or young adulthood and results in lifelong sleepiness and cataplexy, brief episodes of muscle weakness that are similar to the paralysis that occurs during REM sleep. Much of our current work focuses on mouse models of narcolepsy because mice lacking orexins also have sleepiness and frequent episodes of cataplexy. We hypothesize that orexins normally stabilize the activity of wake-promoting brain regions, but absence of orexins produces behavioral state instability, with rapid transitions from wakefulness into sleep, and intrusions into wakefulness of REM sleep elements such as cataplexy or hallucinations. Our major goals are to identify the neural mechanisms through which the orexin system controls sleep and wakefulness and to determine how loss of the orexin peptides results in sleepiness and cataplexy.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/65924

Shafi, Mouhsin, M.D., Ph.D., Associate Professor of Medicine
My research focus is translational research evaluating the relationship between brain function and cognition in human subjects, and investigating and modulating brain function, excitability, connectivity and pathology in patients with neuropsychiatric disorders, typically by utilizing neuroimaging-guided noninvasive brain stimulation techniques such as Transcranial Magnetic Stimulation (TMS) and Transcranial Current Stimulation (TCS) in combination with cognitive testing and electrophysiologic tools such as EEG. My research is oriented towards the application of these techniques to develop new tools to understand and improve human cognitive function, and to improve the diagnosis, management and treatment of patients with neuropsychiatric disorders. I have ongoing projects focused on Epilepsy, Alzheimer’s Disease, post-operative Delirium, sports-related concussion, cognitive aging, surgical skill acquisition, and in better understanding the mechanisms and effects of neuromodulation techniques.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/22825

Siddiqi, Omar K., M.D., Assistant Professor of Neurology
Dr. Siddiqi’s laboratory has been involved in research to understand the disease burden of central nervous system opportunistic infections in HIV-infected Zambian adults. His laboratory uses molecular diagnostics and post-mortem tissue samples to classify the etiology of meningitis and encephalitis among HIV-infected Zambians. More recently his work has focused on evaluating the performance of point of care diagnostics for tuberculous meningitis. He has also been involved in a cohort study over the last 10 years establishing the causes of new-onset seizures in HIV-positive children and adults in attempt to determine risk factors for the development of epilepsy. He helped to start the first neurophysiology laboratory and neurology training program in Zambia. He is also one of the founding members of the Zambia Institute of Neurological Care, Research, and Education (ZINCARE).
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/13329

Simon, David, K., Ph.D., M.D., Professor of Neurology
The Simon lab focuses on the study of the pathogenetic mechanisms that lead to the onset and progression of Parkinson’s disease (PD) and Alzheimer’s disease (AD), as well as translational studies of potential neuroprotective strategies. With a translational approach, they aim to identify, characterize and target faulty cellular mechanisms that lead to neurodegeneration. They study molecular mechanisms of cell death using in vitro and in vivo models of PD and AD. Their ultimate goal is to develop and validate novel strategies that could slow or stop the pathogenetic processes of these neurological diseases. Currently the work focuses on 3 areas. One is VPS35, a gene encoding for a subunit of the retromer complex, which regulates multiple pathways for degrading dysfunctional proteins, including beta-amyloid and alpha-synuclein. Mutations in VPS35 are a rare cause of familial PD. We have found that a pharmacological stabilizer of VPS35 leads to potent neuroprotection in an alpha-synuclein based mouse model of PD. We are further studying the potential for targeting VSP35 in mouse models of AD. A second line of work focuses on USP30, which is a deubiquitinating enzyme that inhibits mitophagy, which is a cellular process for preferentially degrading dysfunctional mitochondria. Considerable data links impaired mitophagy to PD. Accordingly, they have studied USP30 knock-out mice and find that loss of USP30 leads to protection against alpha-synuclein toxicity in a mouse model of PD. A third line of work focuses on pathways regulating PGC-1alpha. PGC-1alpha is a transcriptional coactivator that coordinates mitochondrial biogenesis and anti-oxidant defenses. PGC-1alpha activity is low in dopaminergic neurons in early PD and PGC-1alpha is thus a promising therapeutic target in PD.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/76810

Tian, Feng, Ph.D. Member of the Faculty of Neurology
Dr. Tian lab’s focus is molecularly and computationally dissecting the genetic programs that determine the cell death/survival fate of neurons in neurodegeneration. Addressing this key research question will provide insights into novel neurodegenerative treatments by reprogramming susceptible neurons to be more resistant to disease-related stress.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/168982
ORCiD: 0000-0001-8965-8973

Westover, M. Brandon, M.D., Ph.D., Landau Professor of Neurology
Dr. Westover’s research aims to help people preserve and enhance brain health. They are affiliated with Harvard Medical School, Beth Israel Deaconess Medical Center, the McCance Center for Brain Health at Massachusetts General Hospital, MIT and the Broad Institute. Their research areas include sleep, dementia, epilepsy, delirium/encephalopathy, anoxic brain injury, status epilepticus, the ictal-interictal-injury continuum, subarachnoid hemorrhage, and the neurophysiology of critical illness.
Additional information can be found at the research links below:
http://cdacneuro.org/
http://bdsp.io/
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/84202
ORCiD: 0000-0003-4803-312X

Vanderhorst, Veronique, M.D., Ph.D., Associate Professor of Neurology
Dr. Vanderhorst’s active projects focus on brainstem circuit nodes that are relevant for therapy resistant motor and non-motor symptoms which affect patients with movement disorders and Alzheimer’s disease and related dementias. Examples are gait and balance disorders and sleep disordered breathing. State of the art mouse models using opto- and chemogenetic tools, calcium imaging, electrophysiology and translational behavioral assays serve to unravel circuit function. Parallel research lines employing post-mortem brainstem specimens and clinical data from elderly adults provide insights in the involvement of homologous circuit nodes in a spectrum of neurodegenerative diseases and aging.
Harvard Catalyst Profile: https://connects.catalyst.harvard.edu/Profiles/display/Person/24835
ORCiD: 0000-0002-4089-5393